"Mastering Thermodynamics: Part 1 - Fundamentals, Laws, and Processes"
Table of Contents
Hello friend we are going to start the new subject of rac this the
Topic to be covered
- Definition of Thermodynamic
- Basic terms in Thermodynamic
- Laws of Thermodynamics
- Thermodynamic Processes
DEFINITIONS OF THERMODYNAMICS
The science that encompasses the study of energy, its transformation and relationship amongst the various physical quantities of substance which are affected by cause these transformations.
BASIC TERMS IN THERMODYNAMICS
System :-
Prescribed region of space or finite quantity of matter surrounded by an envelope called the boundary.
Closed System, open system and isolated system
Closed system:-
Open system:-
Isolated system:-
Surrounding :-
The space and matter external to the thermodynamic system and outside boundary is called the surrounding.
Universe :-
When system and the surrounding are put together it is called universe.
Work :-
Heat :-
Heat is defined as the form of energy that is transferred across the boundary of a system at a given temperature to another system (or the surrounding) at a lower temperature by virtue of temperature difference between the two systems. in this heat is deal with the W and KW it is not deal with J and KJ.
Property :-
Measurable characteristics describing the system that depends on the state of the system and is independent of the path (that is, the prior history) by which the system arrived at the given state.
• Intensive Property
• Extensive Property
intensive property :-
Which are independent of mass of system. for example temperature is constant in room. intensive property is temperature, pressure, specific entropy.
extensive property :-
State :-
Unique condition of the system, at an instant of time, described by its properties.
Process :-
Path of succession of state points through which the system passes during transition from one state to another state is called a process.
Cycle :-
When a system in a given initial state goes through a number of different changes of states or processes and finally returns to the initial state, the system has undergone a cycle.
LAWS OF THERMODYNAMICS.
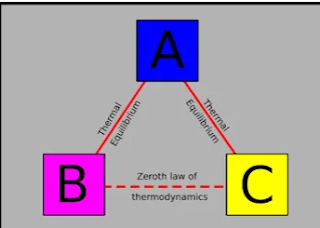
Zeroth law
First law
Second law
Zeroth law
Second law of Thermodynamics or
Qualitative low / Direction Law or
law of degradation of energy
Kelvin planks :-
Clausius statement :-
Thermodynamics process for closed system
- Isothermal process
- Isobaric process
- Isochoric process
- Polytropic process
- Adiabatic process
- Isentropic process
- Isothermal process:- an isothermal process is a type of thermodynamic process in which the temperature T of a system remains constant: ΔT = 0.
 |
- Isobaric process :- during which the system’s pressure does not change.
- Adiabatic process :- is a type of thermodynamic process that occurs without transferring heat or mass.
- Isentropic process :- an idealized thermodynamic process that is both adiabatic and reversible.







Enjoy reading the article above, it really explains everything in detail about Commercial Appliance Repair San Antonio, the article is very interesting and effective. Thank you and good luck with the upcoming article
A very delightful article that you have shared here. Your blog is a valuable and engaging article for us, and also I will share it with my companions who need this info,Evaporative Air Conditioning In Melbourne Australia Thankful to you for sharing an article like this.
This is very educational content and written well for a change. It's nice to see that some people still understand how to write a quality post.! commercial kitchen service restaurant equipment repair.
It is a proficient article that you have shared here.commercial hvac repair phoenix I got some different kind of information from your article which I will be sharing with my friends who need this info. Thankful to you for sharing an article like this.
We have been pleasantly shocked to discover numerous slot themes, able to take you to completely different worlds, even while you’re vegging out on the couch! Explore historic Greek mythology, make a journey to Egypt, or go back to classic casinos with those fruity sevens. Determine competitive salary ranges, compare worker compensation with market benchmarks, and get prompt access to reliable salary survey knowledge online. Check out ERI’s Salary Assessor to get differential pay for night shifts, day shifts, and swing shifts. Using ERI’s sturdy database, you'll be able to|you possibly can} accurately worth jobs based on industry-specific shift differential policies. ERI compiles 카지노 사이트 shift differential pay knowledge based on salary survey knowledge to assist customers discover reliable compensation info.
You have a genuine capacity to compose a substance that is useful for us. You have shared an amazing post about Air Conditioner Installation Service in Portland.Much obliged to you for your endeavors in sharing such information with us.